A Master Plan to Solve the Kidney Transplant Crisis
Kidney failure occurs when our kidneys are unable to filter waste products from our blood. Currently, around 100,000 patients in North America alone experience kidney failure and need to undergo a kidney transplant, which is the ultimate solution for the patient. A kidney transplant is a surgery that replaces a failed kidney with a donor’s kidney that is compatible with the patient’s body. However, because of the limited supply of donors and hence donor's kidneys, only around 20% of all patients on the waitlist get a transplant within the first year, which leaves the majority of patients to wait an average of 3-5 years to a lifetime for a suitable kidney. In the meantime, patients are often put on costly, ineffective kidney dialysis treatments that filter the blood using a machine with 15% efficiency, without curing kidney failure.
The steps that a patient has to go through from the start to the finish of a typical kidney transplantation process is demonstrated below:
There is a critical need for a solution that addresses the issue of unreliable access to kidneys from kidney donors, which effectively causes significant delays in kidney transplantations and forces patients to undergo ineffective and expensive dialysis treatments while they wait years on end to get the transplant.
The Master Plan
To tackle this issue, a business model was developed with the goal of producing bio-ink and 3D bio-printers that have the potential of creating fully functional 3D bio-printed kidneys. We will manufacture and sell the bio-inks and the 3D bio-printers to hospitals so that they can print viable kidneys for their patients when required. Once the US FDA Center for Devices and Radiological Health Medical Board accepts the testing of this product, it will be patented. It is in our best interests to provide this medical device for kidney transplant hospitals in order to save the lives of several people who pass away as a result of the lengthy transplantation procedure.
1. Target Market
Our chosen target market is hospitals specializing in kidney transplantation. These hospitals are more familiar with the kidney transplantation process and may be keen to improve waiting time and transplantation numbers. Since about 84% of patients with kidney problems eventually seek help from these specialized hospitals only, targeting these hospitals will allow us to meet the needs of many patients. Thus, our product will be most used by a hospital that conducts the most kidney transplantation and is willing to generate more revenue. Normally, for one kidney transplantation procedure, patients on average pay $300,000-$400,000, so hospitals can increase revenue by ‘producing more kidneys using our bioprinting technology’ and allowing for more transplantation access to meet the huge demand gap.
2. Value Proposition
The product we are manufacturing and selling will be our 3D bio-printers designed to print transplantable kidney organs & the bio-inks necessary in the process. Our 3D Bioprinters could produce a fully functioning kidney with our specialized bio-inks within 12 hours. Doctors inject cells of patients in the bio-ink, insert it into the printer and wait until a kidney is manufactured live, specific to that patient ensuring no cell rejection. It will be immediately ready for transplantation.
The core benefits for hospitals are: reduce the number of waitlisted patients and waiting periods for kidney organs from 3.6 years to possibly weeks or days, reduce costs related to dialysis treatment, improve timeliness in treating patients, and increase the availability of spaces in hospitals to serve other patients.
The actual product is a 3D bioprinter that is similar to most printer sizes which makes it convenient to place in hospital rooms. It is designed to be user-friendly through the integrated touch-surface screen and can incorporate patient information with our bio-ink to create compatible individualized organs. As the organ is being constructed, the progress will be updated on the screen. As more organs get printed, hospitals will have to purchase our bio-ink cartridges to replenish their supplies. Each bio-ink cartridge has enough materials to help produce 100 kidneys.
The augmented product includes instructions on how to use the technology through the company website and our customer service representatives.
Due to the risk often associated with new technologies, the product’s benefit will be emphasized through the packaging and associated services with regards to this product. The risk presented with new technologies can also relate to the lack of information provided about them. Hence, in addition to our augmented product, a variety of scientific journals and reports on our website detailing the cost-effectiveness, potential efficacy, and safety of our product over long-term usage will be provided. This information will be useful in deciding on candidate technologies, especially because of the complex decision-making process done by hospitals.
Our product and future products will undergo human clinical trials. In addition, there will be a Premarket Approval (PMA) submitted under the FDA as our products sustain life, categorized to be a Class 3 device. The success of developing partnerships with well-known hospitals such as Mayo Clinic will lead to greater trust and adoption of our technologies in other hospitals.
Visualization of the 3D Bio Printing Process
3. Pricing Strategy
For pricing, we took into consideration mainly 3 aspects:
Firstly, we looked at the Price Elasticity of Demand in healthcare in general and it was found to be very low at -0.17, hence it is "Price Inelastic". This applies to both patients seeking medical aid and hospitals purchasing medical equipment. This goes to show that everyone in the value chain will still purchase despite high prices.
Next, we observed the market standard for revolutionary tech in the medical field, such as MRI, CT Scan, X Rays, and Life Support, range from $1 million-$3 million. This gives us a direct understanding of how modern medical fields price effective products and how hospitals still have to purchase them to meet the general standard.
Finally, we observed the current 3D Bio Printing Prices and it was seen that the current printers can only make non-functional prototypes of kidneys (mainly used by doctors for surgery training or educational purposes) for more than $200,000 with inefficient bio-ink prices at over $2,000.
Now, these results along with our value-proposition-based demand, call for the implementation of Price Skimming and Odd-Even Strategy tactics allowing us to price our products as such:
3D Bio-Printer - $1,499,999
Bio-Ink - $14,999
4. Timeline and Costing
The approximate timeline and major costing involved in the process from start of design synthesis to sale of product has been shown:
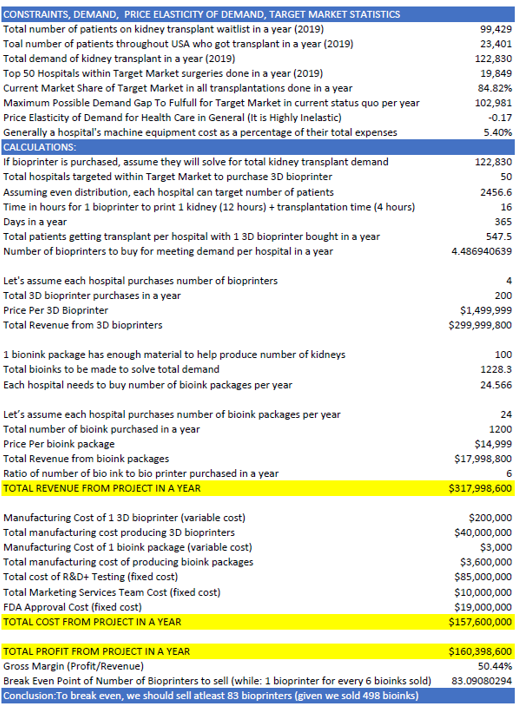
5. Projections and Break Even Analysis
A cash flow statement was created and a break-even analysis was done as shown in the table below. To summarize, it states that we need to manufacture 200 bio-printers and 1200 bio-inks yearly to meet the annual waitlist demand. However, only purchasing 83 bio-printers and 498 bio-inks (roughly 40% of products) will cause a break even with our fixed costs.
Had there been all products purchased in the first year of business, there would be a projected revenue of $318 million and costs of $158 million (Variable: $44 million, Fixed: $114 million), giving a gross profit of $160 million.
The break-even point of units sold was found by dividing the total fixed costs ($114,000,000) by the difference between the price of the unit sold (1 bio-printer and 6 bio-inks = $1,589,993 million) and variable cost (1 bio-printer and 6 bio-inks = $218,000). This gives the value of 83, which indicates 83 bio-printers and 498 bio-inks should be sold for break-even.
However, for the break-even time analysis, conservative assumption calculations were done based on our initial 15 medical sales representatives (MSR) hired, where they can reach 15 hospitals per month, and assuming a very conservatively low 5% conversion rate, around 1 hospital per month will buy 1 3D bio-printer (and 6 bio-inks). Then, we will be able to sell 12 3D bio-printers/year giving time to break even = 83/12 = 6.9 years ~ 7 years.
This means that once we launch our products by the year 2027, as a very conservative approach, it may take till the year 2034 when our break-even occurs.
Risks in Business Model
(a) If we do not get FDA approval, we cannot launch the product for business.
(b) If we are unable to get IP rights, other companies may copy and be a loss leader. This may be an overstatement since the intricate levels of systems that go into production will be hard to copy, but even if they do, our marketing team will have by now tapped into the target market deeply to create a starting loyal customer base among the hospitals.
(c) With our product being a medical device there are high costs pertaining to hiring a well versed marketing team. However, it is necessary since hospitals often buy equipment from specific medical related sales representatives, so it is important to gain their confidence through our representatives. This relationship is important in this industry because if held well from the start, it can lead to more success in our potential other products.
(d) Focusing on direct channels only will decrease the amount of exposure we can get as compared to other channels. This puts a higher pressure on our marketing team to perform well as it is the only channel we are relying on.